Riskonnect for the Pharmaceutical Industry
Riskonnect software helps you manage risk, comply with regulations, and achieve growth.
The pharmaceutical industry is under constant pressure to make sure critical medications are safe and available, comply with evolving and complex regulations, and protect sensitive data.
Do you have the processes, people, and technology to get in front of risks – while protecting your data and your reputation?

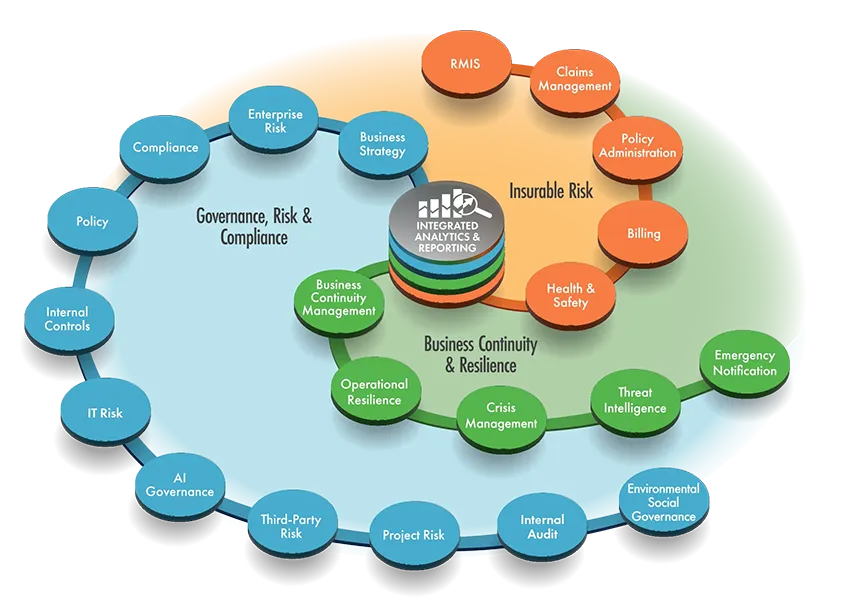
Connect the Dots for Better Results
Riskonnect allows you to seamlessly manage all risk and compliance processes, programmes, and initiatives from one place. The software breaks down silos, automates manual workflows, and improves response times, so you understand what you’re facing, how everything interrelates, and the cumulative impact on the organisation.
Recognised by Leading Analysts




Get more done.
Streamline and automate routine processes, so you can spend your time where it matters most.

Find answers – fast.
See everything you need to identify, manage, and mitigate risk in one, easily accessible place.

Comply with complex regulations.
Simplify compliance by leveraging controls across multiple regulations to satisfy the requirements of auditors and regulators.

Make better decisions.
Identify and respond to evolving risk, stay compliant with global regulations, and make informed decisions.
Every Riskonnect solution is powerful on its own, but the real magic happens when you use them together.
The Riskonnect pharmaceutical
community includes:
life sciences companies